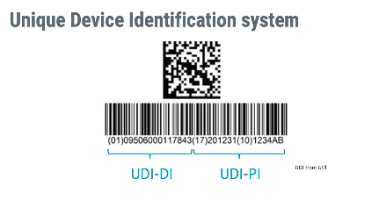
Each health authority creates its own regulations that define UDI and the UDI System, but some parameters have been globally harmonized by the International Medical Device Regulatory Forum (IMDRF) and the standards organizations identified as...
Impinj Gen2X is an advanced, standards-compatible enhancement to the RAIN RFID (GS1 UHF Gen2) standard that improves tag read range, increases inventory speed, boosts security, and lowers overall solution costs. Developed by Impinj, it is designed...
How to Select the correct UHF RFID tag label requires analysing the object's material (metal vs. non-metal), required read range (up to 10+ meters), environmental conditions, and regional frequency (FCC 902-928 MHz or ETSI 865-868 MHz)....
top-selling UHF RFID hardware (readers and printers) is dominated by a few key manufacturers, based on market reports, industry analyses, and sales indicators as of early 2026. The UHF RFID market (especially for readers and printers) continues to...
Using UHF RFID Integrated with AI and IoT - UHF (Ultra-High Frequency) RFID technology enabling long-range, non-contact identification and data capture. When integrated with AI (Artificial Intelligence) for data analysis and IoT (Internet of...
How the integration of Radio Frequency Identification (RFID) technologies with Artificial Intelligence (AI) and the Internet of Things (IoT) transforms traditional tracking into an intelligent, proactive system
. While RFID provides real-time,...
UHF RFID becomes much more powerful when it’s combined with AI and IoT. Instead of just identifying items, it turns into a real-time intelligence layer for physical operations. Below is a structured view of the major implications and...
The combination of UHF RFID (Ultra-High Frequency Radio Frequency Identification, often called RAIN RFID) with IoT (Internet of Things) delivers transformative benefits across industries like supply chain, logistics, retail, healthcare,...
integration of UHF RFID (Ultra-High Frequency / RAIN RFID) with IoT delivers game-changing advantages in the transport and supply chain industries. These technologies shift operations from manual, periodic checks to automated, continuous,...
Integration of UHF RFID (Ultra-High Frequency RFID, also known as RAIN RFID) with IoT (Internet of Things) is particularly powerful for valuable asset management, such as tracking high-value equipment, tools, vehicles, IT hardware, medical...
UHF RFID (RAIN RFID) technologies are experiencing significant growth, with approximately 32 billion labels expected to be deployed in the apparel sector alone
. The market is shifting from simple inventory tracking to advanced automation,...
Using UHF RFID technologies ESG compliance requires transparency, knowing the origin, journey and true cost (environmental and social) of every product, supply chain visibility, often relying on batch-level barcodes or manual checks, simply cannot...
Using UHF RFID technologies ESG compliance requires transparency, knowing the origin, journey and true cost (environmental and social) of every product, supply chain visibility, often relying on batch-level barcodes or manual checks, simply cannot...
How will RFID Technologies and IoT will radically enhance real time data in retail, healthcare and logistics & transport of assets to improve custom,er satisfaction and increase profits
Introduction to RFID and IoT Integration
RFID...
UHF RFID Technology
Ultra-High Frequency (UHF) RFID operates in the 865-868 MHz band in the UK and Europe, offering long read ranges (up to 10-15 meters), high-speed data transfer, and cost-effectiveness for large-scale deployments. It dominates...
Here’s a current overview (UK & Europe) of trends and practical uses of UHF RFID technologies — especially for real-time asset/inventory tracking, theft prevention, operational accuracy, error reduction, and better customer...
The latest trends in RFID Technologies are in UHF (Ultra-High Frequency) RFID, operating frequency in the UK & Europe - ETSI 865.6-867.6 MHz or USA & Canada - FCC/IC 902-928 MHz range, continues to dominate RFID...
How to qualifying a potential for a RFID solution, to uncover use-case, environment, performance, and business constraints. Below is a structured, practical checklist you can use in discovery calls or site surveys—well suited to Auto-ID /...
RFID (Radio Frequency Identification) tags track wooden pallets by using radio waves to automatically log their movement at various checkpoints, providing real-time visibility for delivery and returns management without requiring manual,...
How RFID technology dramatically improves efficiency & Return of Investment (ROI) for Returnable Transport Items
Returnable Transport Items circulate constantly between factories, warehouses, suppliers, and customers. Companies often lose...
Barcode Technologies Ltd launches RFID Technologies web site - www.rfid-uk.com - UK's leading provider, for over 30 years, of products & solutions in barcode & RFID data capture and AutoID and mobile computing systems with...
Enhancing Supply Chain and Manufacturing ffficiencies using RFID Technologies
At RFID Technologies - www.rfid-uk.com, we help businesses transform their supply chain and logistics operations through the power of RFID automation. Our advanced...
RFID UHF (Ultra-High Frequency) technology is used in transport and logistics to enable real-time tracking and management of assets like pallets, crates, and vehicles due to its long read range and ability to read multiple tags at once. It...
How To improve asset inventory visibility in real time with UHF RFID technologies - managing inventory across complex networks is challenging when operations are fast-moving and global and how using RFID Readers and RFID Labels Tags improves...
QR (Quick Response) codes are two-dimensional (2D) barcodes invented by Denso Wave in 1994 to store large amounts of data, including text, URLs, contact info, and payment details, in a small, two-dimensional pattern of black and white squares....
Honeywell QuickCheck QC810 Handheld Linear (1D) Barcode Verifier used to test the quality and readability of 1D barcodes against ANSI/CEN/ISO and traditional standards. It provides a convenient "aim-and-shoot" method for simple...
RFID (Radio-Frequency Identification) technologies is a fast automatic identification system that uses radio waves to wirelessly transmit data between a tag and a reader.
An RFID system typically includes a RFID tag (containing a microchip and...
QR codes barcodes are a perfect example of industrial innovation spilling into everyday life.
They solved a very practical problem on Toyota’s production lines but became a digital bridge between the physical and online worlds.
Their...
Redbeam RFID Tracking Made Easy - Automate, Locate, Streamline & Scale for End-to-end Visibility - from the warehouse floor to the back office, RedBeam is Asset Tracking Made Easy.
Redbeam RFID Tracking Made Easy allows you to gain fast,...
1. Boost Efficiency: Automate Tracking & Eliminate Errors:
Automatic Identification: UHF RFID labels and RFID tags can be read in bulk, without line of sight, as RTIs move through dock doors, conveyor portals, or vehicles.
Faster...
How RFID UHF Technologies used for Returnable Transport Item (RTI) Management brings significant benefits to supply chain operations and logistics and automating workflows, boosts efficiency and cuts costs, enables real-time tracking for complete...
Ideal Use Cases of RFID (Radio Frequency Identification) technology
1. Asset Tracking:
Real-Time Location: Assets like medical equipment, IT devices, or tools can be located instantly across a facility.
Loss Prevention: Alerts trigger if...
HF RFID technologies is the backbone of real-time, enterprise-scale tracking across healthcare, retail, logistics and manufacturing. By automating inventory and asset management while ensuring authentication, it cuts costs, prevents losses, and...
How does integrating RFID technologies with existing barcode technologies in warehouses can achieve a more cohesive and efficient operation, leading to improved performance -n RFID (radio frequency identification) has emerged as a game-changing...
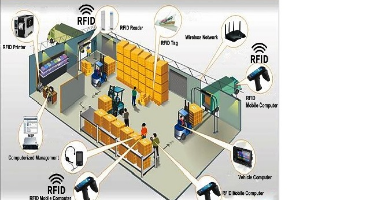
how RFID adoption works in warehouses, logistics, and distribution, and what hardware components must be considered for a successful implementation.
RFID Adoption in Warehousing, Logistics & Distribution
In these environments, RFID...
How RFID adoption works for valuable asset tracking and what hardware considerations are required for a robust installation. Asset tracking is a crucial process for businesses to monitor the location, status, and usage of valuable assets, helping...
How UHF RFID Technologies when applied for Returnable Transport Items (RTIs) like pallets and crates in transport and logistics, and how it actually cuts waste, prevents losses, and boosts profitability.
What We Mean by RTIs - Returnable...
How using RFID UHF Technology for real-time data and visibility for tracking pallets, crates, roll cages, totes used to move goods between suppliers, warehouses and customers in the transport and logistics and supply chain.
Let’s break...
How RFID technology adoption in retail is accelerating right now — a “perfect storm” of pressures from multiple fronts. Let me break it down into a clear, narrative explanation that could work for a report, blog, or...
ow RFID Technology adoption is expanding beyond retail into healthcare, aviation, manufacturing, and other sectors — driven by the demand for accurate real-time data and mission-critical information, with a resulting push for more...
Announcing the launch of RFID Technologies Ltd ( www.rfid-uk.com ) by Barcode Technologies Ltd ( www.barcode-uk.com ) represents a clear, strategic expansion and deepening of expertise in RFID solutions. This new division underscores the company...
BARCODE TECHNOLOGIES LTD (www.barcode-uk.com), the UK’s leading provider of barcode, RFID, Auto-ID, and mobile computing solutions, is proud to announce the launch of its dedicated RFID technology division:
RFID Technologies Ltd –...
Smart RFID Cabinet designed to bring security and real-time asset tracking to your workplace. Using RFID technology and secure user authentication, it ensures that only authorised personnel can access valuable assets tools, equipment, or consumables...
How RFID Technology helps to identify and locate individual assets and items and communicate for stock and inventory management throughout the supply chain management
RFID (Radio Frequency Identification) technology plays a...
How RFID UHF Technology helps to track RFID tagged items as they move through the supply chain, improving planning accuracy, identifying bottlenecks, and increasing supply chain efficiency
RFID...
How RFID UHF Technology helps to manage valuable asset and getting increased real-time visibility into asset management for efficient resource planning track assets as they move through a facility allowing...
How RFID UHF Technology helps to replace manual inventory counting with accurate inventory data is key to keeping an organization efficient and optimized. But many companies are stuck doing things...
How UHF RFID RAIN Technologies delivers an end-to-end RAIN RFID solution to manage inbound and outbound product distribution by streamlining receiving, shipping, and returns processes, delivering complete traceability in real...
How UHF RFID RAIN Technologies helps to combating counterfeits and offers a safe, secure, reliable and scalable method for authenticating critical products as they move throughout the supply chain
UHF RFID...
How RFID UHF RAIN Technologies helps to authenticity and provides privacy, performance, and flexibility while protecting retailers from fraudulent returns
RFID UHF RAIN (Radio Frequency Identification, Ultra-High Frequency, Radio frequency...
How RFID UHF RAIN Technologies in self-checkout in retail benefits inventory management with item-level visibility and real-time inventory tracking, retailers can make faster, more agile business decisions, Sales floor replenishment...
How RFID Technology with the Impinj R720 Enhances Asset Tracking and Warehouse Management
The Impinj R720 4-Port UHF RFID Reader is a high-performance device that allows connection to up to four...
RedBeam real-time inventory tracking software optimizes stock levels and inventory using RedBeam real-time inventory tracking software, follow these strategic steps to improve visibility, reduce waste, and increase profitability and customer...
RedBeam Asset Tracking Software (SaaS) can be highly beneficial for UK businesses in maintaining an asset register and supporting tax compliance, especially in the context of annual purchases of valuable...
How to help customers with RedBeam Web-Based Cloud Real-Time SaaS Asset Tracking Solution with their fixed valauable asset tracking made easy. Eliminate messy spreadsheets, streamline physical inventories better visibility and...
Maximize the benefits of UHF RFID solutions based on its capabilities:
1. Enhance Inventory Management:
Achieve Near-Perfect Accuracy: UHF RFID (Ultra-High Frequency Radio Frequency Identification) technology can improve...
Improving Operational Efficiency with UHF RFID Technology UHF RFID (Ultra High Frequency Radio Frequency Identification) is a powerful solution for boosting operational efficiency across various industries. By enabling real-time visibility...
RFID technology using AI-powered drones can significantly improve warehouse inventory tracking. reducing errors, increasing efficiency and enhancing real-time stock visibility as a valuable tool for warehouses operations
AI-powered drones...
How RFID technology is transforming industries and empowering data identication with manufacturers face increasing pressure to enhance accuracy, ensure regulatory compliance and streamlining operations with complexities and how to solve...
How RFID technology is transforming industries and empowering data identication with manufacturers, heathcare, transport & logistics and valuable assets face increasing pressure to enhance accuracy, ensure regulatory compliance and...
RFID UHF RAIN (Radio Frequency Identification) is an advanced RFID technology that uses Ultra-High Frequency (UHF) radio waves to automatically identify and track valuable assets or people. RAIN RFID is...
RFID UHF RAIN (Radio Frequency Identification) is an advanced RFID technology that uses Ultra-High Frequency (UHF) radio waves to automatically identify and track valuable assets or people. RAIN RFID is...
RedBeam Asset Tracking, hosted on Google Cloud, leverages a robust Software as a Service (SaaS) infrastructure with multiple security layers. This ensures data protection, compliance, and operational reliability while allowing...
RedBeam Asset Tracking hosted on the Google Cloud allows Software As A Service (SaaS) security of this infrastructure is designed in progressive layers including Operational Security, Internet Communication, Storage Services,...
RFID technology delivers business-critical information data throughout the lifecycle of every item manufactured, transported and sold and desposal to helping enterprises gain greater efficiencies, reduce waste and ensure every thing...
RFID technology offers a comprehensive solution for retail organizations to track, manage, and optimize everything they manufacture, transport, and sell. By connecting every aspect of the supply chain, RFID enhances...
RFID technology, particularly using UHF RFID readers and label tags, has revolutionized manufacturing processes by enabling full traceability across production lines. This technology provides significant advantages by ensuring real-time visibility...
RFID UHF technology provides a powerful solution to streamline operations, enhance data accuracy, and deliver full compliance in modern manufacturing and supply chain environments. By integrating this technology into your...
In the field of transportation and logistics, accurate and precision data available plays a crucial role in ensuring smooth operation to delivery. Whether it’s managing goods between warehouses, coordinating deliveries,...
Redbeam Asset Tracking software offers several benefits for organizations looking to improve their asset management processes. Here are some of the key advantages:
1. Improved Efficiency
Streamlined Processes:...
How schools in the UK are using the Redbeam Asset Tracking Software achieve greater control over their assets, leading to increased operational efficiency, cost savings and improved overall asset management
Schools...
How Ultra-High Frequency (UHF) RFID technology, combined with Artificial Intelligence (AI), plays a significant role in modernizing and enhancing supply chain operations across industries globally. Here’s how it contributes to...
What are true values of integrating of RFID and AI technologies RFID (Radio Frequency Identification) and AI (Artificial Intelligence) technology with Quantum Computing will dramatically accelerate faster and more accurate...
Integrating UHF RFID (Ultra-High Frequency Radio Frequency Identification), AI (Artificial Intelligence), and Quantum Computing in retail can significantly enhance automated checkout processes, AI-driven demand forecasting, and inventory...
What are true values of integrating of UHF RFID and AI with Quantum Computing in Manufacturing to accelerate faster and more accurate Real-time asset tracking of raw materials and finished...
What are true values of integrating of UHF RFID and AI with Quantum Computing in healthcare with tracking medical equipment and supplies, managing pharmaceutical inventory and ensuring compliance with regulations...
Benefits of integrating of UHF RFID and AI with Quantum Computing in Logistics & Warehousing with improved inventory accuracy and reduced shrinkage, optimizing warehouse operations with real-time data and AI-driven...
What are values of integrating of RFID and AI technologies RFID (Radio Frequency Identification) and AI (Artificial Intelligence) technology will dramatically accelerate faster and more accurate asset tracking and automated...
How RFID (Radio Frequency Identification) and AI (Artificial Intelligence) technology will dramatically accelerate faster and more accurate asset tracking and automated stock control
Radio Frequency Identification (RFID) and Artificial...
How Radio-frequency identification (RFID) Technologies solves today’s Supply Chain Challenges
Optimism is growing among supply chain professionals now that pandemic-related disruptions are passing. Still, concerns remain, including optimizing...
What is RAIN RFID? RAIN RFID is a passive, battery-free IoT technology that can identify up to 1,000 items per second within a range of a few centimeters to multiple...
What is the Walmart mandate requiring its apparel suppliers to add RFID tags to items so products could be tracked as they were received, distributed, and sold
Walmart has long been...
How Impinj R720 RAIN UHF RFID Reader helps you keep track and monitor valuable assets andf inventory that deliver the flexibility and reliability necessary to provide effective item realtime visibility....
Overcoming operational hurdles with RAIN RFID - Christie Lites knows how to light up a stage. The Orlando, Florida-based global lighting equipment rental company has been illuminating venues — from...
RFID technology has various applications across industries due to its ability to provide real-time tracking and identification of objects. Some common applications include:
Inventory Management: RFID tags are used to track...
Radio Frequency Identification (RFID) technology has revolutionized various industries by providing efficient tracking and identification solutions. Here's a breakdown of its key components and functionalities:
RFID Tags:
RFID Tags are...
RFID technology has found widespread applications across various industries due to its ability to provide real-time tracking and identification of objects without requiring direct line of sight. Here are some...
RFID (Radio Frequency Identification) technology has become increasingly prevalent in various industries due to its ability to efficiently identify and track objects without requiring line of sight. Here's a breakdown...
Frequency Bands: RFID systems operate in different frequency bands, including low frequency (LF), high frequency (HF), and ultra-high frequency (UHF). Each frequency band has its own advantages and is suitable...
You can still buy refurbished Honeywell Handheld Products Quick Check QC810 Barcode Verifier or have your faulty one repaired - Honeywell QuickCheck Verifiers is a full-featured bar code verifier compatible...
A perfect UHF RFID Reader Evaluation Development Kit tool to get started your RFID testing and development of your RFID solution with Nordic ID Medea UHF RFID 1D & 2D...
Honeywell QuickCheck QC810 is a barcode verification device designed to ensure barcode quality according to various industry standards, including ANSI (American National Standards Institute), CEN (European Committee for Standardization), and...
Honeywell Quickcheck QC810 Barcode verification barcode to full barcode quality control to testing traditional and full ANSI/CEN/ISO parameters
Honeywell QuickCheck QC810 is a barcode verifier designed to ensure that barcodes meet...
Honeywell Quickcheck QC810 Barcode verification barcode to full retail barcode quality control to testing traditional and full ANSI/CEN/ISO parameters for supermarket barcode checkour scanning requirements
For supermarket checkout scanning...
Honeywell Handheld QC810 portable or desktop barcode verification enables for simple 1D linear screening to complete ISO/IEC compliance. Barcode accuracy is very important in the supply chain, the Honeywell Handheld...
How mobile RFID (Radio Frequency Identification) UHF (Ultra-High Frequency) readers and barcode scanners indeed offer significant advantages across various industries. Here are some key benefits:
Increased Flexibility: Mobile RFID UHF readers...
How RFID UHF technology is used for Asset Tracking and Management to Track key and valuable assets with attached RFID tags labels in manufacturing, logistics, and warehousing, enabling fixed asset...
Are interested in an RFID (Radio-Frequency Identification) solution for asset tracking using the Impinj R720 UHF RFID Reader, Alien ALR-8698 UHF RFID Antenna, and a UHF RFID Tag sample pack....
Are you looking to changing from a barcode tracking system to RFID tracking system or upgrade your RFID Development Kit, we offfer the perfect ideal RFID Developer Kits includes a...
Impinj R720 UHF RFID Reader Evaluation Development Kit for demonstration, evaluation and development that allows exploration, understanding, determination and development of RFID-based solutions for valuable asset tracking across various industries...
Impinj R720 UHF RFID Reader Evaluation Development Kit, which includes an Impinj R720 RAIN UHF RFID 4-Port Reader, 2 Alien ALR-8698 Antennas, and a range of UHF RFID tags and...
How does Impinj R720 RFID UHF evaluation development kit to provide developers and users with a comprehensive set of tools to experiment with and understand how UHF RFID technology works....
What would the best comprehensive prototyping, testing and developing RFID-based solutionsfor valuable asset tracking solution for developers and users aiming to prototype, test, and develop RFID-based solutions, particularly for valuable...
Zebra new self service EPoS Point of Sale in retail for a faster check-out o more long queues at the till with fast and reliable self service quick check-outs
Nobody...
Tyre-embeddable UHF RFID (Ultra High Frequency Radio Frequency Identification) tags are specialized RFID tags designed to be embedded within the rubber of vehicle tires, particularly in the automotive and logistics...
UHF RFID Tags are integrated moulded into asset in manufacturing for management of individual asset throughout the supply chain.
Integrating UHF RFID tags by molding them into assets during manufacturing is...
RFID UHF technology used for asset on safety components to improve the quality and vehicle safety. detailed data access d on every component relevant the airbags if a vehicle...
How UHF RFID uses to capture valuable data using RFID tags and labels to track trailers, containers and assets within distribution centers and warehouses optimizes inventory control, order fulfilment, transportation...
UHF RFID for work-in-process (WIP) RFID inventory management systems for WIP give real-time information to production control systems, allowing them to monitor and notify every batch and order in process,...
RFID technology for weapon tracking Governments implement best asset tracking practices for their weapon tracking system and Smart City project. RFID UF Label tags are used to create real-time visibility...
How use of RFID technology in yard management logistics processes optimizes inventory control, order fulfilment, transportation management, and warehouse operations, thereby enhancing efficiency and reducing errors real-world use case of...
How use of RFID technology in warehouse management logistics processes optimizes inventory control, order fulfilment, transportation management and warehouse operations, enhancing efficiency and reducing errors real-world use case of managing...
Barcode Technologies Ltd (www.barcode-uk.com) the UK's premier specialist at www.barcode-uk.com, excels in providing innovative barcode, RFID data capture, AutoID, and mobile computing solutions. Our comprehensive suite of products encompasses...
How RFID technologies is used for waste management as local government councils and Municipalities and utilities companies using RFID technology in the field to manage fleets of waste management bins...
How the Impinj M830 and M850 tag chips M800 series RAIN RFID tag chips—next-generation chips that set a new performance benchmark for readability, reliability, and manufacturability will advance Internet of...
UHF RFID (Ultra-High Frequency Radio Frequency Identification) technology has immense potential for businesses of all sizes. By leveraging UHF RFID systems, businesses can overcome the challenges associated with manual work...
How RFID Technology provides real time visibility on supply chain and the increases workforce effieciencies and managing their inventory and ensuring that each store, online and offline, with accurate amount...
RFID technology systems solution using RFID UHF tag labels with RFID UHF Readers will greatly improve inventory management, proof of delivery and operations at manufacturing point to gain visibility all...
Brother TD-2000 2.0inch Wide Label Wristband & Receipt Desk-top Printer is a specialized printer designed for efficient and compliant food labeling. It is commonly used in fast-food chains and other...
using dual-frequency RFID labels/tags for hire rental equipment management offers several benefits over traditional barcode asset labels. Here are some advantages:
Enhanced read range: RFID technology allows for longer read ranges...
How using RFID technology and systems with existing WMS/EPR systems for warehouses play a crucial role in accelerating component and material good inwards, inventory management with put-away location can be...
How Impinj R700 8-port UHF RFID Readers in critical positions and HID LinTRAK RAIN UHF RFID tag sewn into rental linens for tracking and management of high-Volume Laundry for hire...
How using robotic automation systems using Artificial Intelligence (AI), so robots using smart algorithms performing sophisticated pattern recognition based on process data collected directly on the manufacturing and production line...
RFID mandate by Walmart for Item Product RFID Tagging ?
Walmart have specified their RFID mandate for item-level product tagging across its entire inventory. Walmart has been actively promoting the use...
How RFID label tags adhered to each valuable unique ID, enables to identify and locate all at once, from a distance, in real time, providing benefits efficiency and control
RFID (Radio...
How data centres track in real time high value IT asset tracking using RFID label tags for managed IT servers are managed and tracked and to report asset whereabouts proved...
RFID Technology in warehouse forklift picking using RFID UHF tag labels for internal logistics control with WMS and ERP in real-time saves time and money, improves efficiencent and smoother operations,...
RFID UHF tags: Each whisky cask barrel is equipped with an UHF RFID tag. These tags consist of a microchip and an antenna that communicates with RFID UHF Readers.
Tagging the...
RFID Technology improves product supply, engagement and authentication also Track, trace and engage with almost any item or product via a single reliable dual frequency UHF and NFC RFID label...
How RFID Technology using dual frequency UHF and NFC RFID label tags improves product supply, engagement and authentication also track, trace any item or product and speed up inventory management...
How Walmart's RFID Mandate droce to RFID Adoption in Retail and the supply chain and the fuller retail market place ?
Walmart's RFID (Radio Frequency Identification) mandate played a significant role...
Future of warehousing and supply chain and robotics using RFID Technology
The future of warehousing and supply chain, combined with robotics and RFID technology, holds great potential for optimizing operations,...
List of manufactures the world's toughest RFID tags to power Industrial IoT applications in Manufacturing, Aerospace, Oil & Gas, Automotive, and Healthcare.
There are several manufacturers known for producing durable RFID...
How to select the right UHF RFID Mobile handheld readers are widely used in the mobile asset tracking and inventory management and warehouse management and field and sales service ....
How RFID UHF Technology is revolutionizing various industries from retail and healthcare to manufacturing and logistics, empowering organizations to streamline operations, enhance efficiency and drive unparalleled visibility throughout their supply...
How RFID UHF Technology is revolutionizing asset tracking to track and manage valuable assets, such as equipment, vehicles, and tools using RFID tags to assets, organizations can monitor their location,...
How RFID UHF Technology is revolutionizing Pharmaceutical industry to enhance product authentication, anti-counterfeiting measures and supply chain security. RFID tags enable the tracking and verification of medications throughout the supply...
How RFID UHF Technology is revolutionizing manufacturing enables manufacturers to optimize production processes, monitor inventory levels, and enhance product traceability using UHF RFID tagging and tracking items throughout the manufacturing...
How RFID UHF Technology is revolutionizing healthcare industry plays a vital role in healthcare by improving patient safety, asset management, and supply chain efficiency. RFID tags enable tracking and identification...
How RFID UHF Technology is revolutionizing retail sector allows retailers to automate inventory management, improve stock accuracy, and enhance the overall shopping experience using RFID-enabled label tags, retailers can track...
How RFID UHF RAIN technology helps reduce costs and shrinkage in business environments provides Item-level visibility reduces overstocks and markdowns, at the same time providing clear enterprise information to identify...
UHF RFID RAIN technology in retail store operations providing dramatic improvements in real-time item-level visibility and inventory accuracy, with targeted marketing efforts and precise order fulfillment in the supplychain and...
How utilizing RFID UHF Technology for the mining industry to enhance the performance of mobile assets in the field, from maintenance-driven operational efficiency to safety records and compliance
Utilizing RFID UHF...
How RFID UHF Technology for the mining industry to enhance the performance of mobile assets in the field, from maintenance-driven operational efficiency to safety records and compliance drive safety, reduce...
What part will RFID Technology play in the world of Artificial Intellenge (AI )
RFID (Radio Frequency Identification) technology can play a significant role in the world of Artificial Intelligence (AI)...
How Artificial Intellenge (AI ) algorithms ill be using RFID readers and antennas with sensors tags labels will provide global visibility in real-time
Artificial Intelligence (AI) algorithms can leverage RFID readers,...
Radio frequency identification (RFID) systems successfully applied in areas of healthcare organizations in improving patient safety and increasing the impact of it in healthcare.
How Radio frequency identification (RFID) systems successfully...
Nordic ID Medea UHF RFID Adaptive Cross Dipole One 1D/2D Laser Barcode Scanner, WiFi-WLAN a/b/g/n, EU/UK (ESTI)
Nordic ID Medea is designed for quick, accurate and reliable data collection whether it...
How Nordic ID Application platform in use on the Nordic ID Medea UHF RFID Cross Dipole mobile computer reader taking asset inventory and locating products
The Nordic ID Medea UHF...
RFID UHF Technology is used for automation technologies as RFID plays a crucial role in tracking and data collection throughout the entire process work in progress
RFID (Radio Frequency Identification) technology...
How tracking asset in hospitals has direct impact on patient care using an RFID hospital equipment tracking solution provides real-time location of vital of hospital equipment using RFID UHF Label...
What are the bigger challenges facing an RFID implementation in a warehouse and supply chain ?
Implementing RFID (Radio Frequency Identification) technology can bring numerous benefits, but it also comes with...
What are the ideal UHF RFID Tags and labels for an RFID implementation in a warehouse and supply chain ?
For an RFID implementation in a warehouse and supply chain, ideal...
What are the challenges facing an RFID implementation for product Inventory & Asset Management ?
Implementing RFID technology for product inventory and asset management can significantly enhance efficiency and accuracy. However,...
What are the challenges facing a hire company implementing an RFID solution for items/stock hired out and tracking returns and warehouse asset management ?
Implementing RFID technology for a hire company...
What are the latest commonly used UHF RFID technology hardware as well as UHF RFID Tags and Labels ?
Here is a overview of commonly used UHF RFID hardware components...
what is the ideal UHF RFID Tag Labeling Solution
The perfect UHF RFID tag labeling solution depends on various factors, including the specific requirements of your application, the environment in which...
RFID (Radio Frequency Identification) technology in UHF (Ultra High Frequency) has been widely used for various applications, including inventory management, supply chain optimization, asset tracking, access control, and retail operations....
Uses and applications in UHF RFID Technology for real-time allowing to tracking valuable assets using UHF RFID Readers to tracking assets across various locations, such as warehouses, manufacturing facilities, or...
How to improve your return of investment (ROI) using UHF RFID technology to enhance supply chain visibility in real time and tracking goods throughout the entire supply chain logistics processes...
How to utilized RFID technology in Healthcare and Pharmaceutical industry to track in real-time all medical equipment, monitor medication inventory and ensure patient safety. pharmaceutical industry, to help prevent counterfeit...
How to improve your return of investment (ROI) using UHF RFID technology to enhance retail operations for improving product inventory accuracy and enabling efficient omnichannel operations by RFID label tagging...
RFID Tag label selection and placement and RFID label Tag ensuring accurate and reliable tracking for assets or products to be tagged items, environmental conditions and potential interference to help...
RFID technology for Inventory Management and Replenishment for inventory stock management and replenishment processes and real-time inventory data for retailers to automate inventory tracking, monitor stock levels and optimize replenishment...
RFID solution for rental hire companies for asset and inventory management in real-time automated inventory tracking, monitoring assets hired out and accurate returns for correct invoicing
RFID technology can greatly benefit...
Retail barcode scanner for stock taking Nordic-ID RF601 cordless WiFi scanner
Nordic-ID RF601 cordless WiFi scanner is a retail barcode scanner that can be used for stock taking and inventory...
Nordic-ID RF601 Cordless WiFi 1D Barcode Scanner ideal for fast capture barcode data, allowing for efficient prosduct inventory management, stocktaking, and point-of-sale processes.
Nordic-ID RF601 Cordless WiFi Barcode Scanner ...
Nordic ID RF601 Wireless Data Collection Terminal 1D Barcode cordless Scanner ad-hoc repairs and spare parts still available from Barcode Technologies Ltd
Barcode Technologies Ltd offers ad-hoc repairs and spare parts...
Nordic ID RF601 Wireless Data Collection Terminal 1D Barcode cordless Scanner ad-hoc repairs and spare parts still available from Barcode Technologies Ltd
Nordic ID RF601 Wireless Data Collection Terminal is a...
Now available brand new Nordic ID RF601 Wireless Data Collection Terminal 1D Barcode Cordless Laser Scanner RF Terminal Grey uses Narrow Band 433 MHz includes 2 x AA NiMh batteries
Nordic...
How goods selling with RFID labels tags in retail stores and e-commerce operations for both retailers and their logistics operations RAIN RFID greatly improves item-level supply chain visibility and inventory...
Using robot-armed UHF RFID readers in retail shops to scan UHF RAIN RFID tag labels along the shop aisles at night can offer several advantages. Here's how the process can...
How goods selling with RFID labels tags in retail stores and e-commerce operations for both retailers and their logistics operations RAIN RFID greatly improves item-level supply chain visibility and inventory...
How UHF RAIN RFID Tag labels used in supply chain and warehousing using robot armed arones UHF RFID Readers going up and down the warehouse ans stores area aisle at...
Here are some Bluetooth UHF RFID readers that can be paired with tablets, smartphones, and handheld readers:
Zebra RFD90 Sled UHF RFID Bluetooth UHF RFID Reader
Unitech RP901 Wireless Bluetooth Pocket-sized UHF...
A complete guide to an RFID (Radio Frequency Identification) solution involves understanding the technology, its components, implementation considerations, and potential use cases. Here's a step-by-step guide to help you understand...
List of the most used and popular RFID manufacturers
There are several popular RFID manufacturers in the industry that provide a wide range of RFID tags, readers, and related solutions....
How to select the correct UHF RFID hardware
Selecting the correct UHF RFID hardware involves considering various factors to ensure compatibility, performance, and suitability for your specific use case. Here...
How to select the most appropriate UHF RFID software solution partner ?
Selecting the best and appropriate UHF RFID software solution partner is crucial for the successful implementation of your RFID...
How to select the best and appropriate UHF RFID tags and labels - hard and soft tags and labels
Selecting the best and appropriate UHF RFID tags and labels requires considering...
How the use of UHF RFID technology help in medical supplies in hospitals operate some extraordinarily complex and critical supply chains, with RFID enabling automation and new levels of operational...
Using UHF RFID technology in hospitals with challenges of keeping track of medical patient documents and files using UHF RFID label tags enabling elimate errors and efficiency
Using UHF (Ultra-High...
list of top 10 Mobile UHF RFID Reader with 1D & 2D barcode scannerUHF RFID readers with 2D barcode scanners that are highly rated: Zebra MC3390R Integrated Long-Range UHF RFID...
Important considerations factors what UHF RFID Tags used for IT assets tracking RFID for IT asset management On-Metal and off-metal tags and Small or flexible UHF RFID Tags also Printable:...
There are several UHF RFID mobile and fixed-mount UHF RFID readers that can be used for IT asset tracking and management, depending on the specific needs of your organization. Here...
What Mobile RFID Readers should be used for IT assets tracking IT asset management Printed with 2D barcode information on the RFID Tag label surface ?
When it comes to mobile...
Barcode Technologies Ltd is UK's leading provider of products & solutions in RFID and barcode technologies in data capture and AutoID and mobile computing systems, celebrates 28 years being business...
What are the advantages and benefits of using Impinj R700 UHF RFID 4-Port Readers better visibility faster asset management control in warehouse and supply chain for increased visibility, improved efficiency,...
What are the bigger challenges that companies are having installing a UHF RFID Solution ?
Installing a UHF RFID (Radio Frequency Identification) solution can pose several challenges for companies, including:
System Integration:...
What are benefits and limitations to RFID Technology in Healthcare can save organizations time and money, providing real-time traceability, identification, communication, temperature and location data for people and valuable assets...
How can RFID technologies be employed for Automated Unstaffed Corner-Shopping ?
RFID technology can be employed for Automated Unstaffed Corner-Shopping by using RFID tags and readers to track and manage...
what are the huge advantages anf benefits of using RFID Technologies over Barcode Technologies in data capture and autoID in retail and supply chain industries ?
ChatGPT
RFID (Radio Frequency Identification) technology...
What are the return of investment (ROI) using RFID Technologies ?
RFID (Radio Frequency Identification) technologies have been widely used in various industries such as retail, manufacturing, logistics, and healthcare for...
How Does UHF RFID Work ?
UHF (Ultra-High Frequency) RFID (Radio Frequency Identification) is a wireless technology that uses radio waves to identify and track objects or people. UHF RFID operates...
What are UHF RFID Technology appications and uses ?
UHF (Ultra-High Frequency) RFID (Radio Frequency Identification) technology has a wide range of applications and uses across different industries. Some of...
What are the different types of RFID Frequencies ?
There are several types of RFID (Radio Frequency Identification) frequencies, each with its own advantages and disadvantages.
The three main types of...
What are RFID Tags and RFID label ?
RFID (Radio Frequency Identification) tags and labels are small devices that contain an antenna and a microchip that stores data. The antenna receives...
What are different types UHF RFID Readers and how are they to be used and in what applications ?
There are several types of UHF (Ultra-High Frequency) RFID readers that can...
What are the different types UHF RFID Antennas and what specific purposes they atre used for ?
There are several types of UHF (Ultra-High Frequency) RFID antennas that are designed for...
UHF RFID Solution System includes UHF Readers both mobile and fixed-mount, UHF RFID Antennas, UHG Tags labels and RFID cables and RFID software
UHF RFID system typically includes UHF readers (both...
How does RAIN UHF RFID Product Authentication Work ?
RAIN RFID product authentication is a way to verify the authenticity of a product using RAIN UHF RFID technology. Here is how...
How to prevent counterfeit products in pharmaceuticals, luxury goods and electronics using RAIN UHF RFID and Internet of things and savant database Authentification
Preventing counterfeit products in pharmaceuticals, luxury goods, and...
What practical knowledge would be required to qualify, design and deploy a RAIN UHF RFID solution for returnable transport item (RTI) asset tracking in the logistics industry ?
To qualify, design,...
What would be required to deploy a RAIN UHF RFID solution for high value asset tracking solution ?
Deploying a RAIN UHF RFID solution for high value asset tracking requires careful...
How does using RFID drones and RFID robots with RFID Readers and antennas work in the warehouse taking stock take automatically over-night with any interference of the warehouse operations and...
what are Smart UHF RFID Label Tags so-called inlay, made of paper, fabric or plastic.
Smart UHF RFID Label Tags are a type of radio-frequency identification (RFID) tag that is designed...
Uses and applications of UHF RFID technologies in the retail sector using RFID readers, label RFID Tags and automated robots taking stock take and inventory stock and asset management ...
Applications and uses of UHF RFID technologies in the warehousing and supply chain management sector using RFID readers, UHF RFID labels Tags and automated drones taking stock take and inventory...
How does and what is Impinj Authenticity solution engine brings cryptographic authenticity RAIN UHF RFID to verify UHF RFID tagged items with Impinj M775 chip
The Impinj Authenticity solution...
How block chain technology can be used for UHF RFID tagged labeled assets and valuable items for security, traceability and authenticity.
Blockchain technology can be used in conjunction with UHF RFID...
How UHF RFID technolgy can be used for high-value artwork, luxury goods or high-tech components for accurate and auditable record of ownership and for authenticity and value of the assets.
UHF...
Performance: The Cognex Dataman 8700 DX is known for its high performance in reading various 2D barcodes, such as QR codes, Data Matrix, and PDF417, with...
Advantages of using a mobile printer in field and service sales and well retail price updates and retail providing receipts of payments Using a mobile printer in field service sales...
Impinj R400 UHF RFID 4-Port Readers can offer several advantages for better visibility and faster asset management control:
Improved Visibility: Impinj R400 UHF RFID Readers provide real-time visibility into the...
Impinj R700 Antenna 8-port Hub allows upto 32 UHF RFID antennas readers which are compatible with the Impinj R700 RAIN RFID reader, the Impinj R700 antenna hub enables RAIN RFID...
How to selecte UHF RFID hardware products for a planned for a RFID deployment - Selecting UHF (Ultra-High Frequency) hardware products for an RFID (Radio-Frequency Identification) deployment requires careful consideration...
RFID (Radio Frequency Identification) technology can indeed be very useful in managing supply chain logistics in real-time. By using RFID tags, businesses can track their assets, inventory, and shipments as...
What are the advantages and benefits to RFID Android portable terminal technology with 2D barcode reading and long range UHF RFID reading ?
RFID Android portable terminal technology with 2D...
Taking advantange of wearable UHF RFID Readers paired with Android Mobile computers via Bluetooth to deliver and allow freedom of movement with hands-free RFID & 2D Barcode reading
Wearable UHF RFID...
using RFID UHD innovative laundry tracking and trace solutions adds great value for every laundering of textiles, clothing, linens and cleaning cloths can be located at any time in real...
Advancements in UHF RFID technology in the retail industries stock control and management improved costs and cut losses in back door proliferation ensuring that they can meet customer demands while...
Solving the problem of counterfeit products is global, and it’s growing. Knockoff handbags, athletic shoes, wristwatches, and pharmaceuticals cost businesses and consumers billions of dollars every year globally using RAIN...
why and what is migrating to android mobile computers device away from Windows PDAs
There are several reasons why migrating from Windows PDAs to Android mobile computers may be beneficial:
...
Barcode Technologies Ltd in the UK is 28 years old, as a leading provider of products & solutions in barcode & RFID data capture and AutoID and mobile computing systems...
What are the challenges to installation of RFID system in supply chain managers and control of asset and stock management ?
There are several challenges that supply chain managers may face...
Nordic ID RF601 portable WiFi Barcode scanner is a powerful tool for retail businesses looking to streamline their data capture and stock-taking processes. Here are some of its key features...
How to choose the ideal RFID UHF Readers and RFID UHF antenna for warehouse logistics management and stock control
RFID Readers:
The ideal RFID UHF Readers for warehouse logistics management and stock...
Barcode Technologies Ltd offers a full range of wireless solutions, mobile computers PDAs, Barcode scanning equipment, Barcode printers, ID card printers, Barcodes verifiers, Barcode labels, Barcode printer ribbons, RFID printers,...
How RFID technology provides real time visibility for better faster command and control asset and stock management with faster Return of Investment (ROI)
RFID UHF technology provides real-time visibility for better,...
barcode technologies is 50 years old and became the Global Trade Item Number (GTIN), as it revolutionised retail shopping, tracking assets provided better visibility in the supply chain and logistics...
What is RAIN UHF RFID exactly
RAIN UHF RFID is a technology used for identifying and tracking objects using radio waves in the ultra-high frequency (UHF) band. RAIN is an acronym...
RFID Auto-ID solutions in market of asset tracking, Healthcare, Transport & Logistics, Retail, Restaurants, Food Industry, Automotive and Manufacturing
RFID (Radio-Frequency Identification) is a technology that allows for wireless communication...
RFID technology relies on the efficient transmission and reception of radio signals between an RFID reader and an RFID tag. Optimizing antenna placement is critical to achieving maximum performance from...
Digital Product Passport (DPP) is a tool that aims to increase transparency and traceability throughout a product's life cycle. When combined with UHF RFID autoID tracking, it can provide numerous...
How RFID (Radio Frequency Identification) technology has brought a significant change in inventory management and asset tracking in real time in various industries, including the laundry and textile industry.
With the...
There are several reasons why you should seriously consider entering the UHF RFID level with the Printronix Auto ID T800 Enterprise-class Desktop Barcode & UHF RFID Printer.
Here are some of...
Asset tracking: The HH85 can be used to track assets, such as inventory items, equipment, and tools. It allows users to quickly and easily scan RFID...
Passive ultra-high frequency (UHF) RFID technology is widely used in various industries for tracking and identifying items. Some common applications of passive UHF RFID technology include:
Inventory management: Passive UHF RFID...
Inventory Management: RFID technology in the UHF frequency range can be used to manage inventory in large warehouses, retail stores, and logistics centers. By tagging the products with RFID tags,...
RFID (Radio Frequency Identification) technology has been widely used in supply chain inventory management due to its ability to track and manage inventory in real-time. Here are some of the...
There are many different types of RFID hardware available for use in RFID technology, and the "best" hardware depends on the specific application and requirements. Some factors to consider when...
UHF RFID (Radio Frequency Identification) technology is widely used in valuable asset tracking applications because of its ability to identify and track assets over a long range, even in harsh...
Impinj R700 UHF RFID Reader offers fast and highly accurata data and identification of assets, efficiency, durability, and cost-effectiveness make it a valuable tool for businesses looking to improve their...
Automated and faster pick list efficiencies using UHF RFID labels and tags with internal logistics control is still often based on pen and paper and barcode label print-outs only from...
UHF RFID label tags each asset can be given unique identification, that enable you to automatically identify and find locate all at once, from a distance, in real time, without...
Impinj R700 RAIN UHF RFID Reader is designed to meet the needs of high-performance and reliable RFID deployments, especially those that require fast and accurate reading of small, global RAIN...
All new Confidex UHF RFID Tags using the Impinj RAIN RFID tag M780 IC chip for high memory applications. Leveraging the Impinj M780 IC, the new UHF RFID tags offer...
The Barcode turns 70 year old ! What an amazing and brilliant invention - an invention on 7th October 1952, a patent was granted to American inventors Bernard Silver...
Zebra TC72 and TC77 Android Mobile Computer with a 4.7inch High Definition (HD) touch display screen with SimulScan which means you can barcode scan 1D, 2D and Digimarcs barcodes with...
Primary Subsets of Passive RFID - relatively wide range of 860-960MHz is recognized as the ‘Global Standard’ for UHF Passive RFID; however, its late adoption led to the range being...
1D and 2D Barcode Technologies and Radio Frequency IDentication (RFID) Technology operate perfectly together and support each other to enhance and improve asset tracking and tracing solutions
1D and 2D Barcode...
Are you having problems getting fast delivery of your mobile computers for your business in home deliveries, field sales, warehouse and logistics management or stock control and inventory management and...
Datalogic is pleased to announce the launch of the PowerScan 9600 Series, the new industrial handheld scanner developed by Datalogic aiming to address Manufacturing, Warehouse and Retail DIY store applications....
Improve Barcode Verification of 1D or 2D or Direct Part Marked (DPM) barcode to meet industrial Standards and Compliance ISO/IEC 15415, 15426-2, TR-29158 (AIM DPM) with printed quality
Barcode Verification improves...
Create the right Radio Frequency Identification RFID for tool and asset tracking solution for your organization enterprise as we offer and try to share our RFID expertise tried and tested...
IT RFID Data Centers IT Assets Management Solution as data centers start to leverage RFID solutions to automate the quality of service, security and compliance critical to their operations. And...
Zebra TC53 & TC58 6.0" Touch Screen Android Mobile Computers give your workers the fastest speeds with Wi-Fi 6E and 5G. And support for CBRS private LTE networks enables cost-effective...
The barcode verification process differs from barcode reading in several important ways, while barcode readers are designed to read barcode data, a barcode verifier ensures that barcodes are marked correctly...
S01-2D-BT-WiFi enables companies and organisations to easily integrate cordless data capture using 1D and 2D barcode scanning in their business process or service to optimise efficiency. It is lightweight and...
The BCT-1020 is an ultra-thin form factor, 10.0 inch by 10.0 inch and less than an Inch thick antenna thick, This BCT-1020 is compact size of 250mm x 250mm x...
Impinj R700 RAIN UHF RFID Reader is ideally suitable for global RAIN RFID deployments as it delivers excellent read range and rate performance and offers ease of use and deployments...
Radio Frequency IdentiFication (RFID) systems can be broken down by the frequency band within which they operate, there is low frequency (LF), high frequency (HF) and ultra-high frequency (UHF).
There are...
Uses of RFID technology using RFID labels and RFID tags on every asset to item-level tagging of inventory tracking throughout the supply chain offers massive benefits and very fast return...
RFID passive tags and labels bring significant advantages that are ideal for low cost and long lifetime. However, some use cases have differing requirements. In addition to passive RFID, there...
Radio Frequency Identification - RFID technology enables the easy and with a unique identification and tracking of items with the use of a UHF RFID tags that is placed on...
Nordic ID HH85 mobile handheld UHF RFID reader builds on an IP65 and 1.6-meter drop-tested frame which is useful when tracking your assets in difficult environments. The ergonomic pistol grip...
What is Natasha's Law and how will it affect your businesses ? Wekll Star TSP654IISK Thermal Printer is a perfect solution for for Natasha's Law coming into force this October...
For all food labelling showing ingredients in pre-packed for direct sale foods - Barcode Technologies offers a complete labeling solutions to adhere to this law including an easy to use...
Barcode Technologies Ltd is offering a complete labelling solution to businesses for ALL the printing warnings as well as clearly showing all the ingredients used with an EPoS barcode...
deadline nears Natasha’s Law legislation comes into effect October 2021, any food that is prepacked for direct sale and supplied directly to the consumer or to a mass caterer in...
Natasha's legacy becomes law and Natasha’s Food Labelling Law introduced to protect allergy sufferers to provide them confidence and safety in the food they buy to ensure that businesses of...
RFID Software utilises Barcodes and RFID Technologies to uniquely identify items for Asset & Inventory Tracking and Management Software applications Solutions as a Saas.
RFID Software applications which helps run businesses...
RFID Software App software utilises Barcodes and RFID Technologies to uniquely identify items for Asset & Inventory Twracking and Management Software applications Solutions.
RFID Software applications which helps run businesses more...
Data Connectivity and Communication Matter - Provide patient-centric care with purpose-built healthcare technology using Android 9.0 Rugged Smartphone 6.0" Screen Mobile Computer. Ensuring the right patient receives the right care...
Barcode Technologies is offering an excellent Medical Clinical Assistant Mobile Computer Healthcare Smart Android Devices with serious healthcare challenges within the NHS. We offer the vast experience and knowhow to...
There is now a vey severe shortage of semiconductor chip impacting the electronics and the automotive industries and these same IC chip shortages are also impacting the RFID industry including...
Zebra's ALL new Android 10.0 mobile computers MC2200 and MC2700 for retail, manufacturing, warehouse and logistics as well as field sales & service.
They are ideally suited for customers with...
allerge food safety and compliance to protect your customers with Compliant Food Labelling.
Foods such as sandwiches, cakes etc... freshly prepared has now to legally applied labels that include food allergy...
Nordic ID, a manufacturer and supplier of state-of-the-art RFID, barcode readers and solutions appoints Barcode Technologies Ltd UK, a global company of barcode and RFID products hardware and labels and...
Alien ALR-H450 Android Mobile Handheld UHF Gen2 RFID reader as well as the Alien ALR-S350 UHF Passive RFID Sled and the Alien ALR-H460 and H480 Android 6.0 and the Alien...
Track and trace your personnel, assets within an Warehouse Logistics, sales & field or offices for documents or any other environment with an industry established RFID tracking software as a...
New and Innovative RFID technologies provide RFID solutions that will automate logistics and supply chain processes and forward-thinking companies are embracing Internet-of-Things (IoT) technologies and a data-driven approach to drive...
Honeywell RT10 rugged tablet is designed for rapid user adoption, minimized TCO and optimized device management. Whether you’re a supervisor or a shop floor employee, a manager or a maintenance...
Real world applications for uses of RFID are commonly used apparel and retail, supply chain and inventory management (i.e., tires, agricultural, etc.), asset and people personnel tracking (i.e., pallets, containers,...
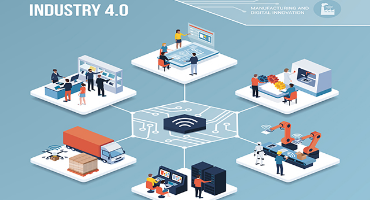
SME companies and industries are under constant pressure to improve equipment maintenance, simplify processes, improve efficiencies and cut downtime. Industry 4.0 unlocks data barriers and positions manufacturers as forward-thinking technology...
Memor 20 is the largest PDA full touch displays in the Enterprise market, thereby maximizing the application user interface, while still in a compact and rugged, yet modern form-factor. This...
Keep constant track of your computers, equipment, furniture, vehicles and all other valuable assets using this comprehensive SaaS cloud-based real-time asset tracking solution. Speeds up your tracking process and improves...
All new cloud web-based real-time Asset Software as a Service (SaaS) Tracking Solution from Redbeam - comprehensive SaaS cloud-based real-time asset tracking solution. Speeds up your tracking process and improves...
Barcode Technologies www.barcode-uk.com delivers a complete RFID Tracking solution for persons and things - assets. A one-stop shop for complete RFID Tracking solution for persons and things - assets to...
Barcode Technologies delivers customer satisfaction for home deliveries with full visibility throughout the supply chain using rugged smart devices for Logistics, Transportation & Warehousing with real time data using rugged...
All-in-One Access Control Solution Non-Contact Auto Identification and Authentication for people and authorised people and personnel and contractors or even paid and authorized spectators or audiances is a standalone module...
Barcode Technolopgies offering most exciting range bespoke, made to order of various RFID Reader to RFID Antenna SMA to SMA and RP to TNC and MCX RFID Cable solutions in...
In these challenging conditions, the reader must perform, and this is where one of the lightest, rugged designs in the RFID market can help. The battery gives you up to...
RFID Systems Solutions employed recently that have paid for itself in just 24 months - Does RFID implementation have to be an extensive operation? You know that implementing an RFID...
RFID UHF Label Tags are adhered to plastic crates and pallets in Industrial and Logistics Solutions Smart supply chain management solutions perfect for Returnable Transport Items (RTI) vastly enhances security...
Zebra TC21 and TC26 Mobile Android Computers offers a large 5.0inch Colour HD touch screen with LED backlight and Corning Gorilla Glass with latest Android 10 operating system and an...
RFID Technologies in action for fast automatic Identification is a critical phase in the manufacturing process. With RFID systems, manufacturers can cover every identification need from the parts in the...
Price checkers can be easily transformed into touchscreen sales tools. Online shopping has made shopping convenient and easily accessible. It allows customers to personalize information once creating an account. Walking...
One typical example of using RFID Tecchnologies in action: Automotive manufacturer Dana Incorporated achieves 100% parts traceability - Leading car part manufacturer Dana Incorporated must have eyes on every item...
RFID & IoT Electronic tags with RFID sensors work to digitally track a product’s location and its purchase habits. Smart shelves usinmg RFID antennas come to life with their tag...
Robust with high IP are emplyed using hand-held Mobile Computers devices that are easy and intuitive to use speed up deployment and keep the employee satisfaction on a high level....
Maybe the price still isn’t right - When RFID burst onto the scene as an upgrade from barcode technology, it seemed too good to be true – and for most...
Through the use of sensor-enabled automated devices - Robots, workers can maintain situational awareness of their workplace environments, by communicating with co-workers remotely, in an effort to limit the face...
BarTender Enterprise Automation editions include 3-options BarTender 2019 Professional and Enterprise and Automation Edition whichh offer complete audit trail capture, allowing you to respond quickly during a recall or site...
Digitally enhanced patient healthcare using leading edge android touch screen tablets and kiosk digital solutions & easy, clean, safe and portable barcode & RFID Labelling solutions to support the Healthcare...
HealthCare Digital Tablet Solutions and HealthCare Handheld Mobile Computers to provide real-time connectivity for the most essential applications. These two, side by side, can scan and capture data from even...
Robots have the ability to sense and avoid people, as well as unobstructed things in the course of completing work. Intelligent Factories using Artificial Intelligence ( AI ), Robotics, Internet...
UHF RFID label tags are an ideal tracking for CryoGenic Ultra Low Temperatures of -200°C (392°F) applications for tracking & Trace data information and records, identifying, locating and delivering fast...
BARCODE TECHLOGIES LTD www.barcode-uk.com is offering RFID Technologies for tracking and tracing samples sent off to laboratories. Of course, BARCODFE TECHNOLOGIES has had to over-come the challenges of samples sent...
With the on-set of a global Pandemic SARS-COVID-19, Barcode Technologies launches a Automatic Hand Sanitizer Wall Mounted Dispenser with Android 21.5" Touch Screen Digital Signage Kiosk for Hospitals, Clinics, GP...
We design and offer complete self-service point of sale EPOS solutions with possibilities andflexibility across environments. Whether you require a fixed-lane point-of-sale or a self-service placing orders solution, we can...
Digital advertising boards on walls with Interactive advertising touch-screens designed to inform and entertain, advertise, inform customers using easy easy to use touch screens. Self-Service EPoS Solutions with innovative Self-service...
Impinj start the mass production of M700 IC chips to be released to the UHF RFID tag & label manufacturers very soon, so that larger IPJ-M730A-A00 and IPJ-M750A-A00 IC Chip...
Impinj M730 and M750 ICs delivers high performance, fast inventory capability with advanced features for next-generation RAIN RFID tags that can be attached to, or embedded in, nearly ALL items,...
Role of RFID Technologies in Warehouse and Supply Chain management to customer at point of sale for just-in-time delivery....
Barcode Technologies launches the ALL new RFID Iron-On Linen and Laundry UHF RFID Tags Label for tracking your linen such as bed sheets and towels, table clothes to name a...
Impinj M730 and M750 tag chips is a game-changer as endpoint ICs (integrated circuits) used in RFID Labels and RFID tags will enhance RFID Technologies in the Industry 4.0 Revolution...
Barcode Technologies www.barcode-uk.com pays tribute to George J Laurer who co-invented the barcode in the 1973...
The Honeywell Quick Check has been dis-continued for over 6-years now and there alot of QC810 verifiers still in use today as they conform the the industry standards ANSI/CEN/ISO and...
Barcode Technologies is now able to fast deliver RFID labels and tags from stock !...
Active RFID Tags are battery or external power operated whereas Passice RFID Tags get their power from the reader RF field...
The first Android Enterprise Recommended device in the rugged space from Datalogic is Memor 10, part of a new family of rugged Android PDA devices, offering a slim and compact...
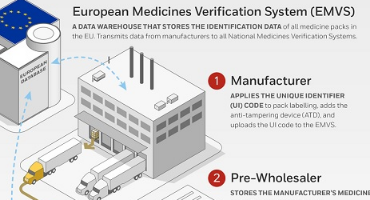
There are only two months left until the European Union Falsified Medicines Directive (FMD) is rolled out. Starting in February 2019 EU citizens will be protected from falsified or counterfeit...
Zebra launches an easy to use RFID Technologies software application utility which allows you to start using RFID Fixed-mount Readers and so now you'll no longer need to be an...
Elo 03 series of screens with 19.0’’, 22.0'', 24.0'' and 27.0 inch touch displays for hospitals and pharmacies for faster and easier administration operations for better hygienic hospitals environments...
How will the Falsified Medicines Directive (FMD) EU directive work delivering medicines from manufacturers to patients in a way eliminate falsified fraudulent counterfeit medicines in the supply chain....
FMD EU directive is a barcode tracking system to track legitimate medicines from manufacturers to patients to eliminate any falsified fraudulent counterfeit medicines in the supply chain....
FMD requires all medicines to be uniquely serialised, protected by tamper-proof seals and their authenticity verified before being dispensed to patients at the point of sale. This can be achieved...
Handheld Products introduce MaxGo, a suite of apps for Handheld Android customers. it’s easy, fast and free - include MaxGo Staging, MaxGo Kiosk and MaxGo KioskBrowser.
...
Using a combination of critically positioned RFID Readers and Antennas with RFID tags and labels as identifiers for AutoID, attached to manufacturered goods and products, IoT & Industry 4.0 and...
Have you aging legecy mobile computer data terminals that are piling up and STILL not repaired ? Not sure what to do with them ? Don't throw away your...
Print your warehouse box item or location barcode labels on-demand and stop wasting time sending staff back and forth to central barcode label printing points !...
Bluetooth allows RFID UHF tags to be read and transmitted to mobile smart phones devices with Android, iOS, Linux and Windows...
Manufacturing processing is undergoing the industry’s greatest change in more than 100 years. In the recent years many domestic jobs have evaporated from many countries with globalization to the East,...
Barcode Technologies is now offering UHF RFID Thermal Transfer Printable Self-adhesive Tag Labels for various applications for item-level Tagging and tracking including asset tracking, supply chain logistics as well as...
This acquisition of Xplore adds to their strategic growth in the mobile computers touch tablets and laptops which includes a portfolio of semi-rugged and ultra-rugged tablets and 2-in-1 rugged laptops...
All high street retail pharmacies shops and stores will have to be European Union (EU) directive compliant with the new Falsified Medicines Directive (FMD) law which comes into effect with...
Innovative Elo I-Series Android 2.0 touch screens Interactive Signage Display Gets Shortlisted as another recognition from the AV Industry, with the I-Series for Android 2.0 selected as AV Awards Finalist...
Handheld Group, a leading manufacturer of rugged mobile computers, today announced that the Nautiz X2 enterprise handheld is now available with the Android 7.0 operating system. The popular Nautiz X2...
Retail EPoS checkout is the perfect environment for the Gryphon products; however, a variety of options and the overall ruggedness of the ALL NEW Gryphon GD4500 2D barcode scanners...
JADAK recently introduced a new product: ThingMagic IZAR – a 4-port UHF finished reader, which serves as a superior replacement of the Mercury6. The ThingMagic IZAR provides significantly better...
Datalogix Falcon X4 mobile Computer achieves best performance times in production and operations in warehousing and logistics in the supply chain, as well as in high street retail....
Zebra provides aggressive regular security/patch updates with smooth migration to the next Android OS with the right security support and you can even obtain 5 to 7 years of security...
Convergence Systems Limited (CSL), a global manufacturer of RFID products and active RTLS equipment, has appointed BARCODE TECHNOLOGIES LTD - www.barcode-uk.com as a distributor Partner to cover Europre for their...
This is an EU directive to harmonised EU measures to fight medicine falsification, ensure all medicines are dispensed safely, and that the trade in medicines is rigorously controlled.
The EU Measures...
Created to prevent counterfeit medicines from entering the European supply chain and this will require pharmacists and pharmacy shops in the UK and Europe to scan the 2D barcodes, check...
Announcement of the launch of a newly revamped aesthetically re-designed website with refined product categories and menu structure for an enhanced user-friendly customer experience in accessing over 50,000+ barcode &...
“End of support” for Microsoft Windows Embedded Mobile Computers ! The year of mobile operating system transition from Window to Andriod O/S ?...