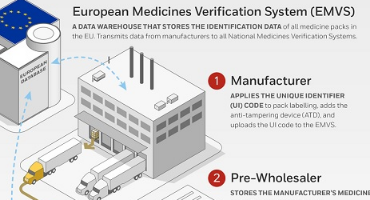
The Pharma Manufacturer
Applies the Unique Identification (UI) 2D Datamatrix barcodes to packaging labeling and adds an anti-tempering device and uploads the UI 2D barcode data to the National Medicines Verification System (EMVS)
Unique 2D DataMatrix barcode, developed to ISO standards with key data elements include:
• Product code
• Unique serial number
• Expiry date
• Batch Number
Pre-wholeseller
Holding facilities and stores the manufacturers' medicines and sells them to a wholesellers
Wholesellers
Will scan and verify the 2D Datamarix IU Barcodes medicines that comes directly from a manufacturer or pre-wholesellers.
Pharmacy and Pharmacists
Will check that the ATD is intact and scans & verifies the FMD UI 2D barcodes using 2D barcode scanners against the National Medicines Verification System (EMVS) database. The medicines is at this point de-commissioned, anf thereby changing it's status in the NMVS from “active” to “inactive”.