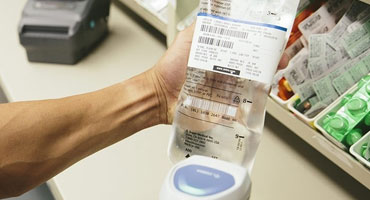
This New directive (FMD) by the EU Compliance now means that 2D barcode scanners have to be installed in all pharmacies to read 2D barcodes to prevent anti-counterfeiting medicines, as costs are in the billions each year and also are extremely dangerous as a health hazard.
All pharmacists and pharmacy shops will have to barcode scan all medicines dispensed at the dispensing and point of sale from February 2019
The new legislation requires pharmacies to provide staff training and start installing barcode scanning equipment, while manufacturers will have to put new barcodes on their medicine packaging.
IT retail systems suppliers will also need to provide pharmacists and pharmacy shops with new barcode scanning software to read and capture the secure barcode data.
The 2D barcodes scanners listed here will read/scan the barcode data that manufacturers will include on packs of medicines.
The EU Falsified Medicines Directive (FMD) and What It Means
Europe’s Falsified Medicines Directive (FMD) comes into full force on 9th February 2019 is a mandatory requirement for product coding, traceability and tamper-evidence will be enforced in over 30 European countries.
Europe’s Falsified Medicines Directive (FMD) requires that all unit-of-sale pack of prescription medicines must carry a “safety feature” includes a 2D Data Matrix barcode and human readable data and must be also tamper-evident.
The 2D datamatrix barcodes must contain a minimum of four pieces of information: batch number, expiry date, global trade item number (GTIN) and a randomized serial number.
The data must also be printed in human readable form, ideally adjacent to the code.
Manufacturers will barcode their products and report encoded barcode data to a central EU Hub run by the European Medicines Verification Organisation (EMVO). This will push barcode information data down to appropriate national data repositories run by corresponding National Medicines Verification Organisations (NMVOs).
High street pharmacists shops and stores will be able to scan these 2D barcode the point of sale and at the dispensing process as these codes will get checked against the local databases as not a counterfeit & falsified medicines and therby closing the counter checking loop.